At Northwestern University, engineers have made a new type of graphene* electrode: one punched with many microscopic holes. It could store 10x as much power (30,000mah instead of 3000) and charge 10x faster. As with any new technology, a big problem is to make it cheap enough for consumers to buy.
* Graphene is a single layer of carbon atoms, usually on a substrate. "Graphite" is actually many layers of graphene.
Source
Stanford Extreme Tech. 2011.
Issues and studies on health, food industry, food politics, and sustainable ideas.
How to print these articles
There are several methods to print these articles.
Using notepad or text editor:
Select text you want to print, copy it to the clipboard using CTL-C. Paste into notepad or your favorite text editor using CTL-V. Use your text editor to print.Monday, April 24, 2017
Monday, April 17, 2017
Monsanto faces more new cancer lawsuits
Monsanto is facing more lawsuits, and a bundle of 3 lawsuits was filed in St. Louis recently. The claim is glyphosate exposure caused them to get non-Hodgkins lymphoma, for which there is some correlation to glyphosate exposure.
This graph of correlation shows how closely the studies found glyphosate exposure was related to certain illnesses. Non-Hodgkins lymphoma was the most correlated.
To make this graph, many studies were reviewed. Dots on the right hand side of the graph show more correlation of glyphosate exposure to cancer. NHL has the most studies, with the most correlation to glyphosate exposure.
Source
Ecowatch.
This graph of correlation shows how closely the studies found glyphosate exposure was related to certain illnesses. Non-Hodgkins lymphoma was the most correlated.
To make this graph, many studies were reviewed. Dots on the right hand side of the graph show more correlation of glyphosate exposure to cancer. NHL has the most studies, with the most correlation to glyphosate exposure.
Source
Ecowatch.
Monday, April 10, 2017
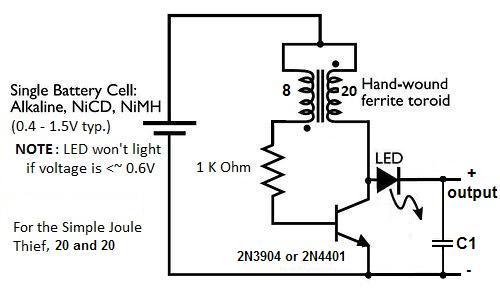
Joule Thief circuits
A Joule Thief is a circuit that can boost voltage. It can be useful to make LED flashlights, or for emergencies, where you only have a single 1.5vdc battery. The Joule Thief (JT) can be used to boost the 1.5vdc to 3.0vdc or more. All the parts can be bought at Radio Shack, or Ebay.
The below Joule Thief is very efficient. It can be made from simple parts and is powered by one AA battery (or any 1.5v battery).
This Joule Thief needs 0.4v-1.5vdc as input. You will have to make your own custom toroid though.
Below is another simple JT circuit.
The below Joule Thief is very efficient. It can be made from simple parts and is powered by one AA battery (or any 1.5v battery).
This Joule Thief needs 0.4v-1.5vdc as input. You will have to make your own custom toroid though.
Below is another simple JT circuit.
Thursday, April 6, 2017
DIY batteries and choosing an electrolyte
When making DIY batteries, which electrolyte should we use? It should be easily found in stores, inexpensive, and fairly safe. But which liquid electrolyte works best?
copper metal | copper sulfate solution | salt solution | zinc metal
This uses 2 different paper layers soaked in each electrolyte: copper sulfate and a salt solution. The problems with this is the paper would eventually dry out unless it is in a sealed container. Putting shrink tubing around a penny and zinc battery might help.
But ethanol, ammonia, vinegar, sulfuric acide, and muriatic acid can also be used. But saltwater can be very harsh on certain metals like magnesium.
By looking for a "galvanic potential" chart we can see that aluminum and graphite in a solution of hydrogen peroxide and salt can work well, but the hydrogen peroxide will not last long, especially if exposed to light.
Source
http://www.pa.uky.edu/~sciworks/em/preview/voltz.htm
More examples of electrolytes, including bases.
The best electrolyte we found was hydrogen peroxide + table salt. Hydrogen peroxide is available at drug stores and safe to use, but we suspect the solution has to be made up fresh -- it won't keep.Another battery they talk about uses 2 different electrolytes, one electrolyte for each metal:
copper metal | copper sulfate solution | salt solution | zinc metal
This uses 2 different paper layers soaked in each electrolyte: copper sulfate and a salt solution. The problems with this is the paper would eventually dry out unless it is in a sealed container. Putting shrink tubing around a penny and zinc battery might help.
But ethanol, ammonia, vinegar, sulfuric acide, and muriatic acid can also be used. But saltwater can be very harsh on certain metals like magnesium.
By looking for a "galvanic potential" chart we can see that aluminum and graphite in a solution of hydrogen peroxide and salt can work well, but the hydrogen peroxide will not last long, especially if exposed to light.
Source
http://www.pa.uky.edu/~sciworks/em/preview/voltz.htm
More examples of electrolytes, including bases.
Monday, April 3, 2017
Broken energy meters report up to 600% more energy used than actually used
A new study by the University of Twente in Holland shows many meters can give inaccurate readings, in some cases showing up to 582% more energy used than was actually used. The most problematic meters are the newer digital "smart" meters. Older meters with a rotating disk are more accurate.
Subscribe to:
Posts (Atom)